Integrate Analytics as needed.
All samples need to be analyzed – regardless whether sampling was performed manually or automatically. As data integrity requirements continue to increase, automated consolidation of protocolled test conditions and analytical results into one report drive the implementation of integrated systems.
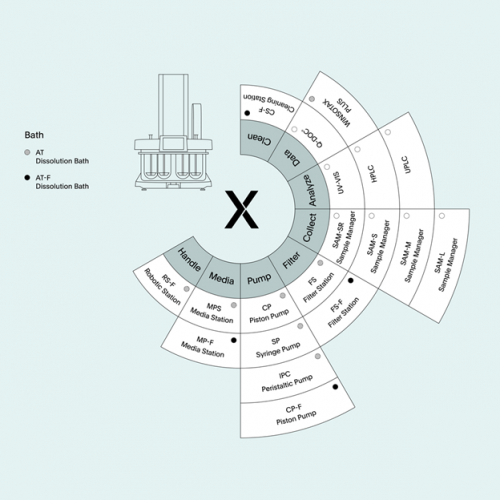
- Your Data Simply Managed - Actively managing your dissolution data saves costs and ensures regulatory compliance.
- EasyTouchTM Intuitive stand-alone control – The EasyTouchTM user interface is designed for fast and intuitive operation.
- q-doc® All your data in one place – Take full control of your data and manage all your methods, results, and users with q-doc®.
- Configure your XtendTM System – Thanks to its modular design, all XtendTM components that are in contact with your product are always 100 % identical – irrespective of your system’s auto- mation level.
- Automate Sampling Integrate Analytics – Take your samples the same way every time. Automated sampling with identical withdrawal positions ensures repeatability – and allows for fast sampling timepoints pushing through fine filtration even when using surfactants.
- Share Modules double capacity - Double your throughput and execute two dissolution tests with one semi-automated system. Available for all analytical configurations, double systems* allow simultaneous sampling from two dissolution baths into one sample manager or one analytical device.