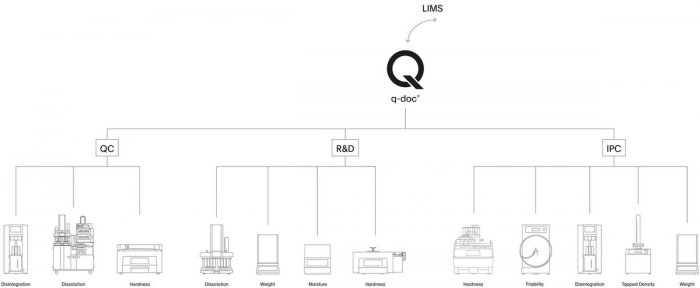
q-doc® – Data Management Software
ALL YOUR DATA IN ONE PLACE.
Take full control of your data and manage all your methods, results, and users with q-doc®. Use the same method on different systems, consolidate data from multiple test runs in a single report, and avoid redundant management of users and their passwords. From electronic signatures, audit trail, and advanced user management to LDAP integration, batch comparison, and LIMS import / export functions – the q-doc® framework is designed for implementation of an efficient, fully 21 CFR Part 11 compliant system.
- Powerful framework with device-independent methods that can be used for different instrument types
- More than 35 drivers for dissolution and physical testing instruments
- Report, check & evaluate data directly in q-doc and sign with electronic signatures
- Easily create complete batch reports and compare batches over time intervals
- Human readable audit trail and product version control for full traceability and compliance
- Fulfills all requirements for implementation of a 21 CFR part 11 compliant system